1.3. 1: Energy in Systems
Energy in all systems is subject to the Laws of Thermodynamics.
According to the First Law of Thermodynamics: Energy is neither created or destroyed. What this really means is that the total energy in any system including the entire universe is constant all that can happen is that the form the energy takes changes. This first law is often called the law of conservation of energy.
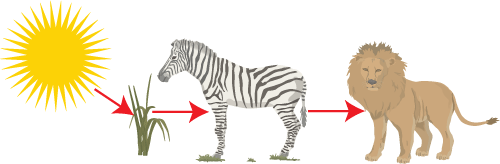
In the food chain above the energy enters the system as light energy, during photosynthesis it gets converted to stored chemical energy (glucose). It is the stored chemical energy that is passed along as food. No new energy is created it is just passed along.
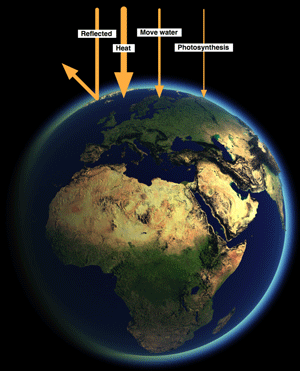
Even if we look at the sunlight falling on Earth not all of it is used for photosynthesis.
30% is reflected, around 50% is converted to heat, and most of the rest powers the hydrological cycle - rain, evaporation, wind, etc. Less than 1% of incoming light is use for photosynthesis.
The Second Law of Thermodynamics states that the entropy of an isolated system not in equilibrium will tend to increase over time. what this really means is that the energy conversions are never 100% efficient: When energy is transformed into work, some energy is always dissipated as waste heat.
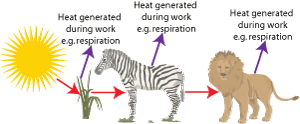 If you examine the food chain again in terms of the second law then: when the lion chases the zebra, the zebra attempts to escape changing the stored chemical energy in its cells into useful work. But during its attempted escape some of the stored energy is converted to heat and lost from the food chain.
If you examine the food chain again in terms of the second law then: when the lion chases the zebra, the zebra attempts to escape changing the stored chemical energy in its cells into useful work. But during its attempted escape some of the stored energy is converted to heat and lost from the food chain.
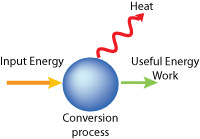
This process can be summarized by a simple diagram showing the energy input and outputs.
The Second Law can also be thought off as a simple word equation:
ENERGY = WORK + HEAT (and other wasted energy)
So what does the term ENTROPY mean?
Entropy refers to the spreading out or dispersal of energy. Using the above example the energy spreads out - the useful energy consumed by one level is less than the total energy at the level below - energy transfer is never 100% efficient.
Depending on the plant their efficiency at converting solar energy to stored sugars is around 2%. Herbivores on average only use around 10% of the total plant energy they consume the rest is lost in metabolic processes and a carnivores efficiency is also only around 10%.
So the carnivores total efficiency in the chain is 0.02 x 0.1 x 0.1 = 0.0002
This means the carnivore only uses 0.02% of incoming solar energy that went into the grass. The rest of the energy is dispered into the surrounding environment.
An interesting if bit heavy explanation of Thermodynamics in ecosystems by Dr James Kay of the University of Ontario